This problem has been solved. This gas law allows you to do calculations for situations in which only the amount of gas is constant.
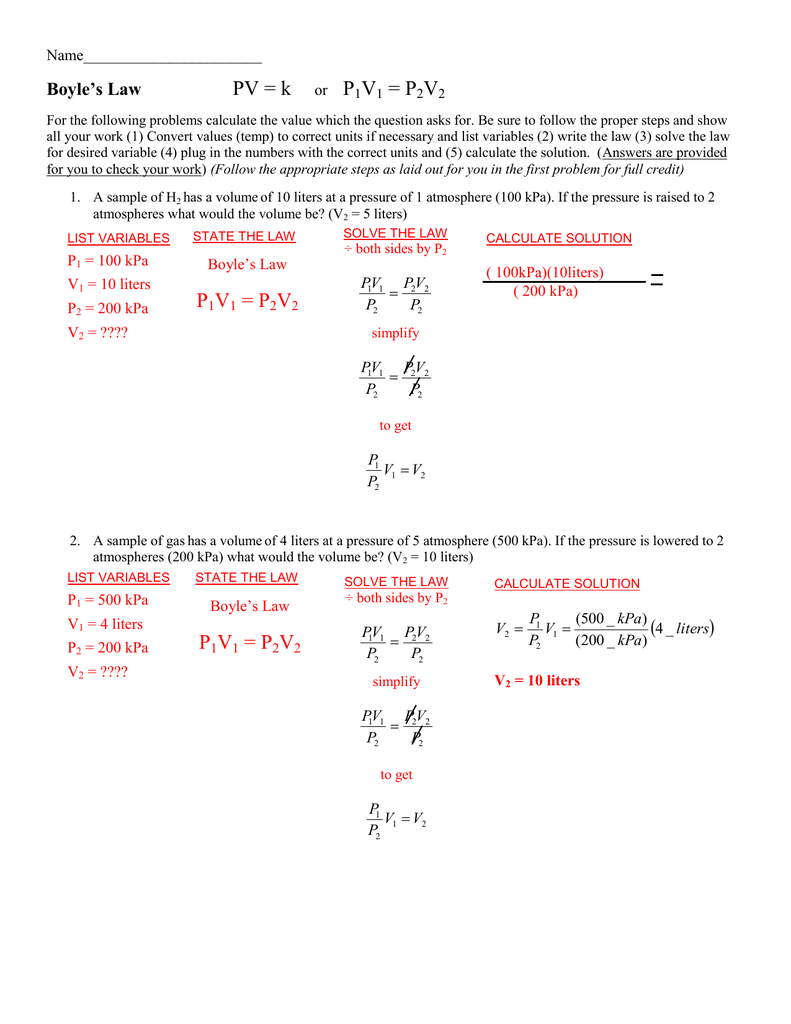
Pv K Or P1v1 P2v2 P1v1 P2v2 P1v1 P2v2
Gay-Lussacs Law Charles Law Avogadros Law Boyles Law.
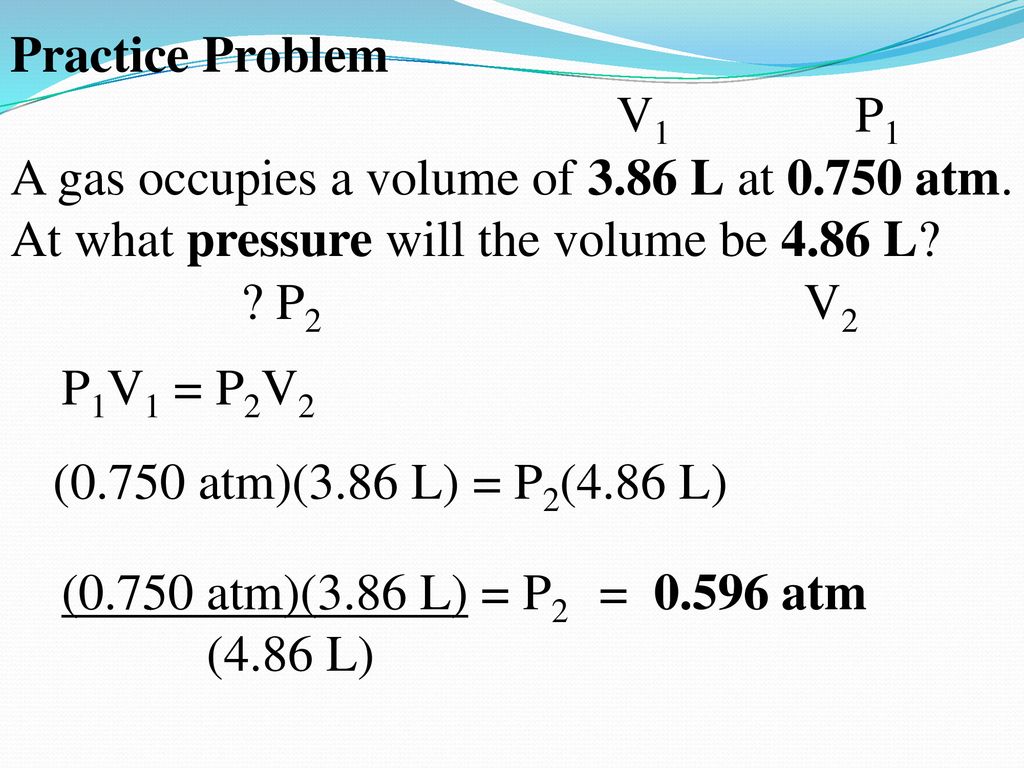
. If p2 02 bar then we have V2 p1V1p2. What law is described by the equation P1V1 P2V2. W I N D O W P A N E.
Boyles Law is described by the equation P1V1 P2V2. In an equation form. In the equation the figures for temperature T pressure P and volume V with subscripts of one are representative of the initial condition.
For a given amount of gas the product pV is a constant -. This problem has been solved. Boyles law states that pressure and volume have an inverse relationship.
Need help with chemistry. Boyles Law is described by the equation P1V1 P2V2. If a given amount of gas is compressed it occupies a smaller volume if the gass available volume is increased the pressure of the gas decreases.
Which simple gas law is represented by P 1 V 1 P 2 V 2. As per the combined gas law. Be notified when an answer is posted.
Which simple gas law is represented by P1V1P2V2. From Boyles Law we have p1V1 p2V2. P1V1 P2V2 where P1 and V1 are the initial pressure and volume values and P2 and V2 are the values of the pressure and volume of the gas after change.
25 6273 P2 3200. Want this question answered. Added 1022018 84321 AM.
V1 T1 V2 T2 GayLussacs Law. This answer has been confirmed as correct and helpful. The relationship for Boyles Law can be expressed as follows.
The combine gas law states that the ratio of the product of pressure volume and absolute temperature of a gas is always a constant. Equation PV K Where P pressure V Volume K Is the constant. See the answer See the answer See the answer done loading.
Ah well thats the behaviour described and measured by Robert Boyle. See the answerSee the answerSee the answerdone loading. Terms isothermin thermodynamics a curve on a p-V diagram for an isothermal process.
The Ideal Gas Law define the law described by the equation. Confirmed by Sting 1022018 85037 AM 34872477. For a fixed amount of an ideal gas kept at a fixed temperature pressure and volume are inversely proportional.
Where P1 and P2 Initial and final pressure of the gas V1 and V2 Initial volume and the final volume of the gas. P1V1 P2V2 Charles Law. Also those with the subscripts of two are representative of the final condition.
The volume of an ideal gas is inversely proportional to its absolute pressure at a constant temperature. So substituting in the formula. P1V1T1 P2V2T2 Temperature must be in Kelvin and the pressure and volume have to be in the same unit on both sides.
Added 1022018 84911 AM. PVK P1V1P2V2 April 2 2020 Boyles law Boyles law states that the pressure of an ideal gas increases as its container volume decreases. Correct option is A Boyles law.
General Chemistry Boyles Law P1V1P2V2 Example 1 - YouTube. V1 n1 V2. Sh hare your windo w.
This answer has been confirmed as correct and helpful. Since the balloon bursts at 02 bar pressure the volume of the balloon should be less than 1135 L. V2 1 bar x 227 L 02 bar 1135 L.
P1 T1 P2 T2 Avogadros Law. If p1 is 1 bar V1 will be 227 L. Which law can be used to explain pressure volume temperature moles and universalideal gas constant as well as molar mass and mass.
Starting pressure times starting volume equals ending pressure times ending volume. Mathematically the law is represented by P1V1 T1 P2V2 T2.

Boyle S Law Y A X Pressure A Volume Pv Constant P1v1 P2v2 Ppt Download
Explain Boyle S Gas Law Qs Study

Boyle S Law Practice Problems Examples Explained P1v1 P2v2 Youtube

0 Comments